Contact Us
Apnimed
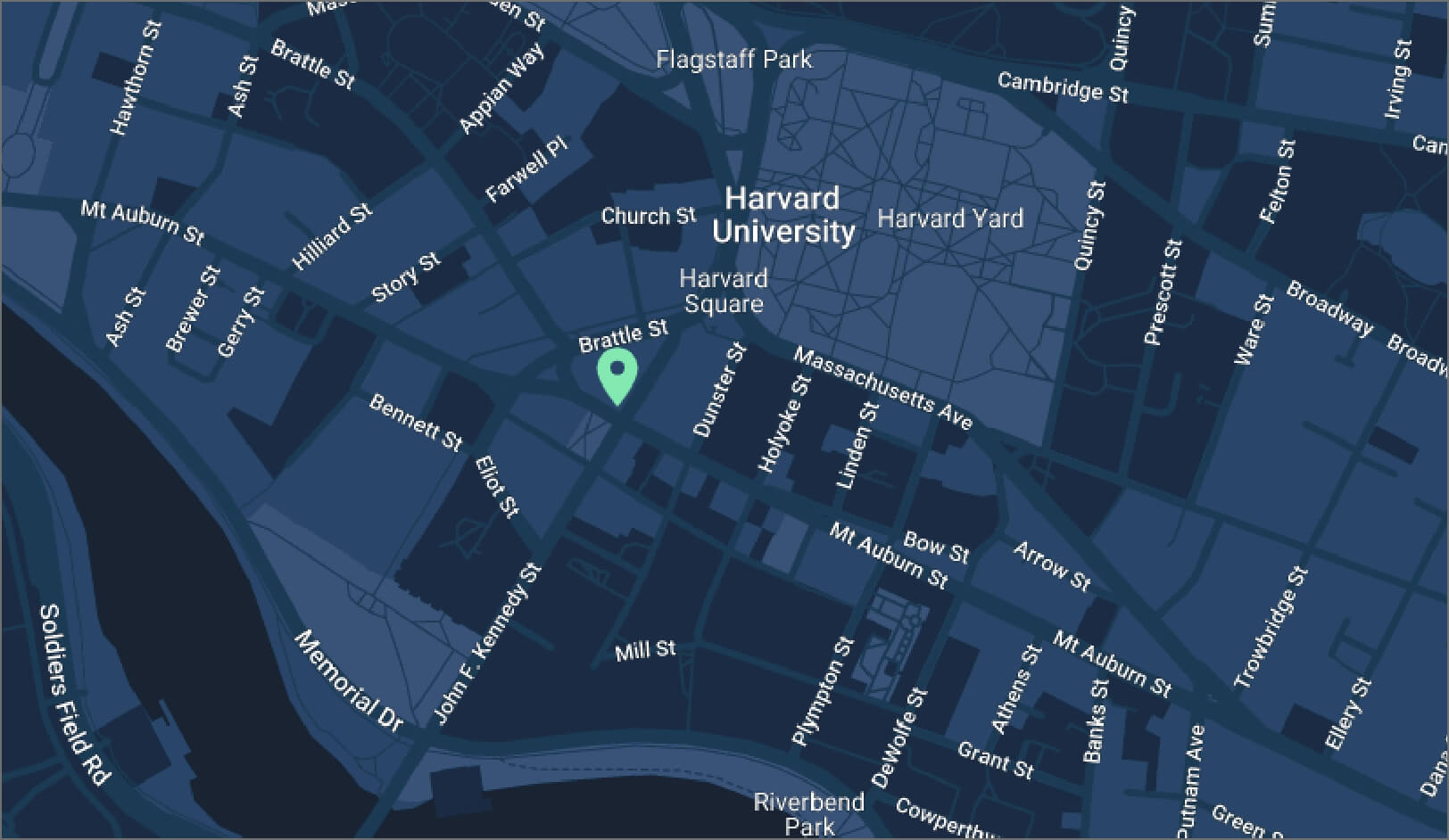
39 John F. Kennedy St., 4th Floor
Cambridge, MA 02138
1-877-APNIMED (1-877-276-4633)
contact@apnimed.com
Get directions
Investor Relations
Media
Medical
1-877-APNIMED (1-877-276-4633)
Landmark Topline Phase 3
AD109 Data in OSA
AD109 Data in OSA
Read More