

Clinical Trials
Through our clinical development program, we are striving to transform the lives of people living with sleep-related breathing diseases. Our product candidates are designed to be a more convenient, oral alternative to current treatment options.
Why enroll? Enrolling in an Apnimed clinical trial offers the opportunity to help advance obstructive sleep apnea (OSA) research and treatment options to potentially benefit others living with OSA.
Ongoing Trials in the AD109 Phase 3 Clinical Program

Visit Open Label Continuation Protocol for OSA on clinicaltrials.gov
Completed AD109 Phase 2b and Phase 3 Studies

Visit LunAIRo on clinicaltrials.gov
View LunAIRo Phase 3 Topline Results

The SynAIRgy trial is a Phase 3, randomized, double-blind, placebo-controlled, 6-month parallel-arm study of AD109 vs placebo in participants with mild to severe OSA. Topline results of this trial were announced on May 19, 2025, highlighting that AD109 met the primary endpoint, mean change in apnea-hypopnea index (AHI, p=0.001) at 26 weeks, across all weight classes and a broad range of people with mild, moderate and severe obstructive sleep apnea.
Visit SynAIRgy on clinicaltrials.gov
View SynAIRgy Phase 3 Topline Results

Apnimed has completed MARIPOSA, a Phase 2b randomized, double-blind, placebo-controlled, parallel-arm, dose-finding clinical trial that evaluated AD109 as a possible treatment for OSA in a broad range of OSA patients over a 1-month period. The study results showed that AD109 achieved a statistically significant reduction of AHI compared to placebo (P<0.001 vs placebo). Dosing with AD109 led to clinically important reductions in AHI in most patients with mild, moderate, and severe OSA. MARIPOSA results also showed that AD109 improved daytime fatigue, an often-debilitating side effect of OSA.
For Additional Information:
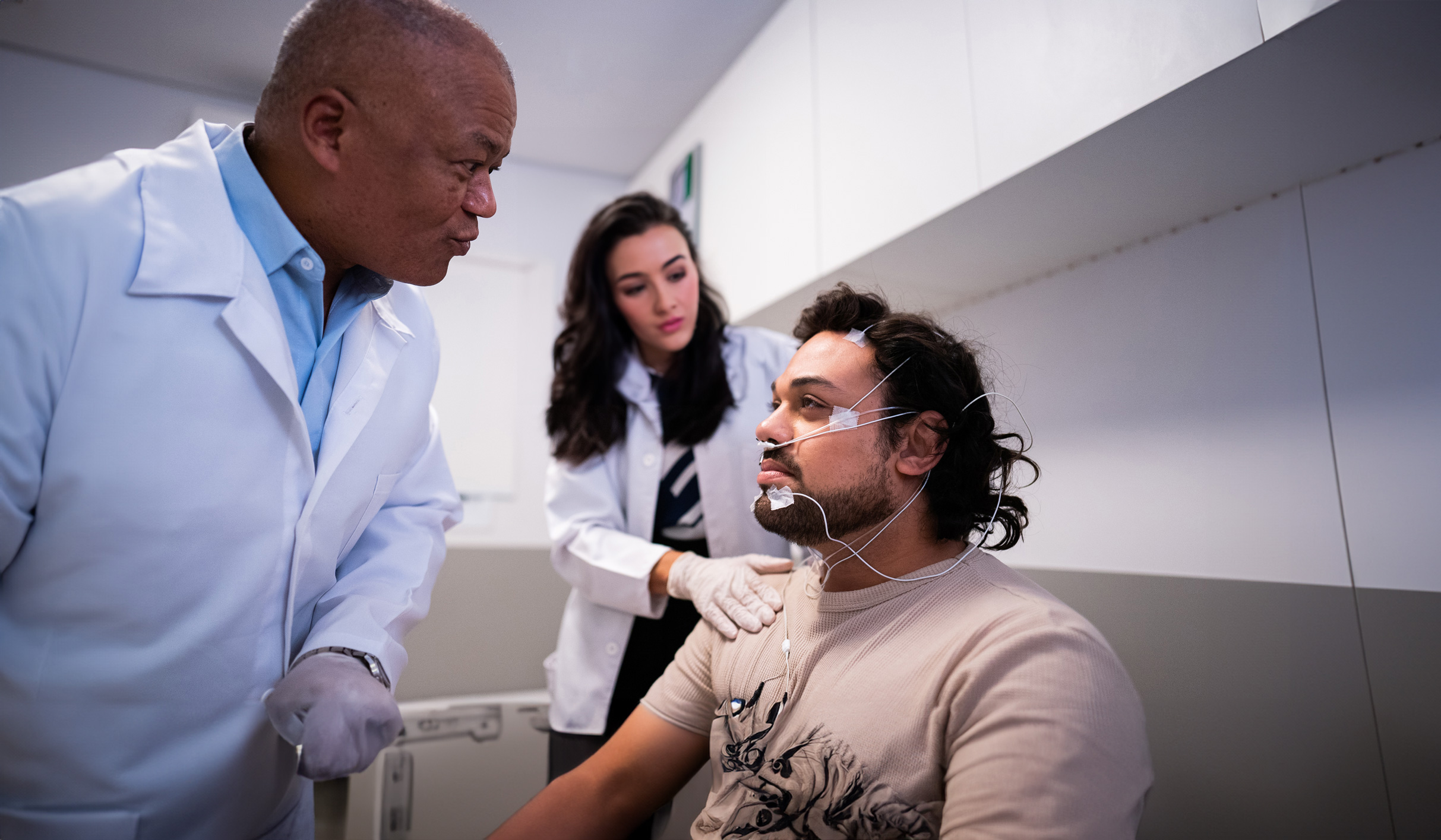

Clinical Trials
FAQs
Clinical trials are an important research tool that advance medical knowledge and patient care. These trials help to assess the safety and effectiveness of potential new medicines and would not be possible without the participation of volunteers.
What is a clinical trial?
A clinical trial is the process used to collect data to study the effects of an investigational medication.
All medications must be reviewed and approved by the regulatory authorities (in the U.S., the Food and Drug Administration) before they can be prescribed to people who need them.
This review process is conducted to learn more about the investigational medication and ensure that it is safe and effective for the intended population.
There are four phases in the clinical trial process. The first three phases occur prior to regulatory approval of an investigational product, while the fourth phase serves to monitor real-world performance of the product after regulatory approval. Apnimed’s MARIPOSA study is a Phase 2b trial, and the LunAIRo and SynAIRgy studies are both Phase 3 trials.
Who can participate in a clinical trial?
Each trial has specific eligibility criteria that outline the factors for those who can and cannot participate. Some of the criteria can include age, gender, medical conditions, current medications, and the stage of a particular disease. These conditions are known as “inclusion criteria” and “exclusion criteria.”
It is important to note that inclusion and exclusion criteria are not used to reject people personally. Instead, the criteria are used to identify appropriate participants for the trial based on what is known, to date, about the investigational medicine, and are intended to help keep participants safe.
What happens when an eligible participant decides to join a clinical trial?
During the first study clinic appointment, the participant will be provided with an informed consent form. The document includes important information about the clinical trial, including study procedures and potential study risks. The participant will review the informed consent form with a member of the study team and will have a chance to ask questions about the study. If the participant agrees to the information in the document, they will sign it before participating in the trial.
The participant will then be asked to schedule one or more screening visits with the study team. During the screening visits, the doctors and staff members will conduct a variety of tests and assessments to determine whether or not the participant meets all of the eligibility requirements. If the participant meets all requirements, they can participate in the study.
The participant will be expected to attend study visits and undergo clinical trial procedures as outlined within the informed consent form.
For the duration of the trial, the clinical study team (doctors, nurses, and other healthcare professionals) will follow the participant’s condition closely.
All clinical trial participation is voluntary. The participant can withdraw from the trial at any point in the process.
Can I get access to Apnimed investigational drugs through expanded access or compassionate use?
Apnimed is a clinical stage pharmaceutical company committed to identifying, developing, and commercializing life-changing therapies for people with OSA. People who choose to participate in our clinical trials play a critical role in helping develop our investigational therapies. While we do not offer investigational therapies outside of our clinical trials, you may determine that enrolling in a trial may be an option for you. For more information about Apnimed’s ongoing clinical trial programs, including whether enrollment is currently open, please review our open trials on clinicaltrials.gov.
Expanded Access Policy
We are a clinical stage pharmaceutical company focused on developing novel oral pharmaceuticals to treat sleep-related breathing diseases. Our proprietary approach recognizes that OSA is largely a neurological disease, and AD109, our lead product candidate, is an investigational oral therapy designed to target the key underlying neurobiological mechanisms that lead to airway obstruction.
Expanded access refers to the use of an investigational drug outside of a clinical trial when the primary purpose is to diagnose, prevent, or treat a serious condition in an individual who lacks available therapeutic alternatives. Expanded access is different from a clinical trial in which the primary purpose is to collect extensive safety and efficacy data to support submission of an application to the U.S. Food and Drug Administration (FDA) to market a drug.
Apnimed believes that the most appropriate way to access our investigational products is by participating in our clinical trials, which are managed by medical experts and are designed to determine whether the investigational products are safe and effective. Therefore, Apnimed is not accepting expanded access requests at this time. Apnimed’s clinical trials help evaluate the mechanism of action, safety, and efficacy of these investigational products. Our trials are designed to help ensure that important safety and efficacy monitoring practices are in place, which both help protect individuals and ensure that product development is proceeding as quickly as possible. People who are interested in participating in one of our trials are encouraged to discuss their specific needs with their physician. Information regarding our ongoing trials can be accessed at clinicaltrials.gov.
In accordance with the 21st Century Cures Act, this policy may be revised at any time. Should you have additional questions, please reach out to your physician, or email contact@apnimed.com. We typically will respond to inquiries within 7 business days.


Explore Our Pipeline of Programs
Check on the progress of our entire product portfolio.


Stay Updated
Submit your email for Apnimed company news, including updates on AD109.