

Who We Are
Apnimed is a pharmaceutical company dedicated to breathing new life into the sleep-related breathing disease treatment landscape through innovation in oxygenation.
Deep expertise fuels our team’s pursuit of solutions, giving us a unique scientific, clinical, and commercial edge. Born out of research from Brigham and Women’s Hospital in Boston, our lead candidate, AD109, aims to revolutionize the treatment of obstructive sleep apnea (OSA) as the first oral therapeutic.
Apnimed envisions a new era where novel oral therapies simplify intervention, expand the reach of diagnosis and treatment, and elevate the health and expectations of everyone in the sleep-related breathing disease community.
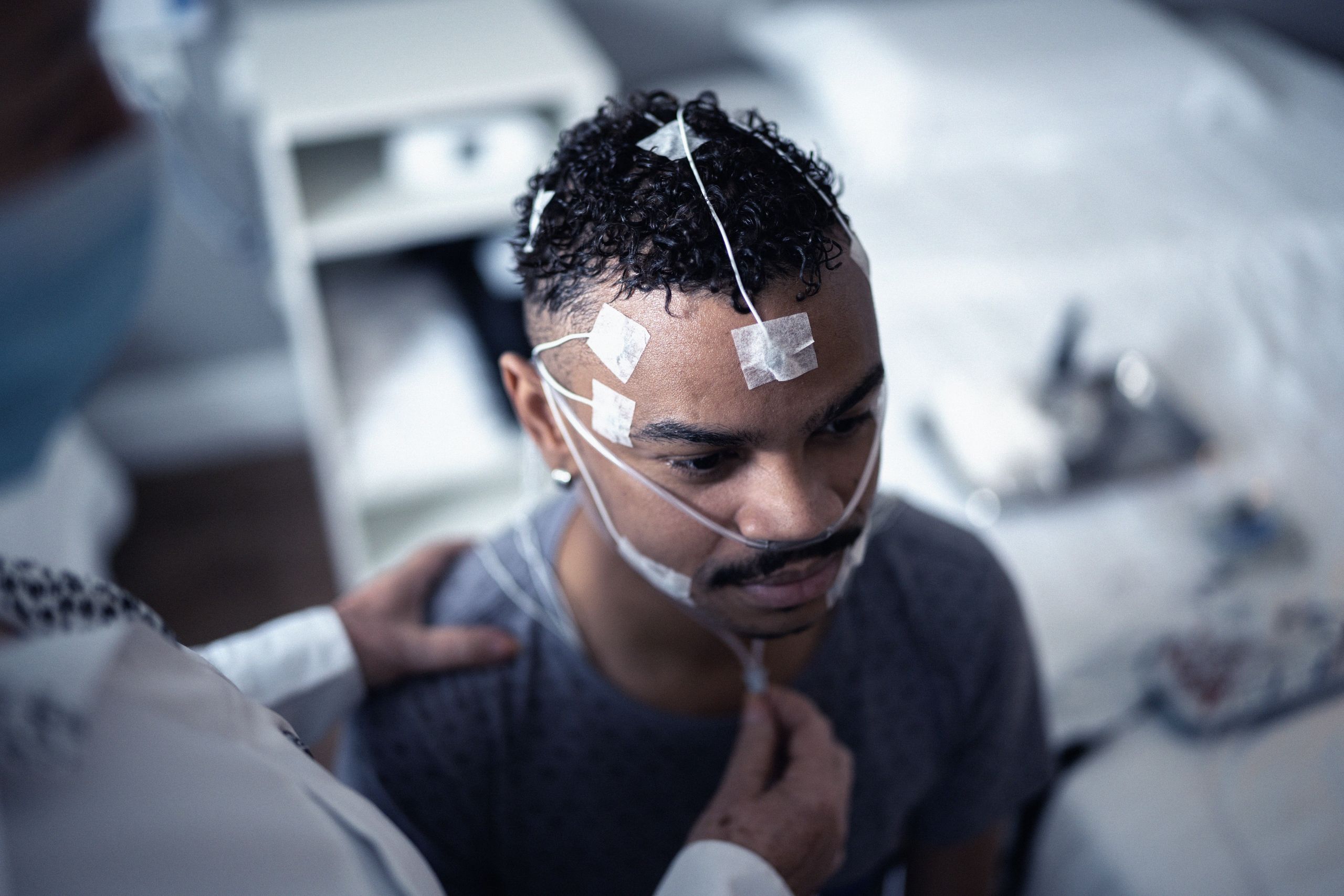

Committed to the Community:
The Apnimed Way
Just as sleep-related breathing diseases profoundly disrupt lives, we are driven by a shared purpose to disrupt the treatment paradigm to make positive change happen.
For those who feel unseen and overlooked, we believe everyone with a sleep-related breathing disease deserves restful nights, vibrant days, and improved health through simple, safe, and effective therapies.

I feel so fortunate to work with a team who is so committed to addressing the needs of the OSA community
Ketan Mehta
Head of Product and Engineering, Connected Wearables


Explore Our Clinical Pipeline
We are advancing a portfolio of oral therapeutic candidates for sleep-related breathing diseases.


Apnimed Leadership
Our executive team stands boldly for people living with OSA.
AD109 Data in OSA
Read More
































































