

AD109: The Novel Neuromuscular Approach In Development to Improve Oxygenation and Address Unmet Need
If approved, AD109 could be the first pharmacological treatment to improve oxygenation during sleep by directly addressing the underlying neuromuscular cause of upper airway collapse in people with obstructive sleep apnea (OSA). No mask or device would be needed—the potential power of AD109 has been developed in a pill.
The Synergistic Science of AD109
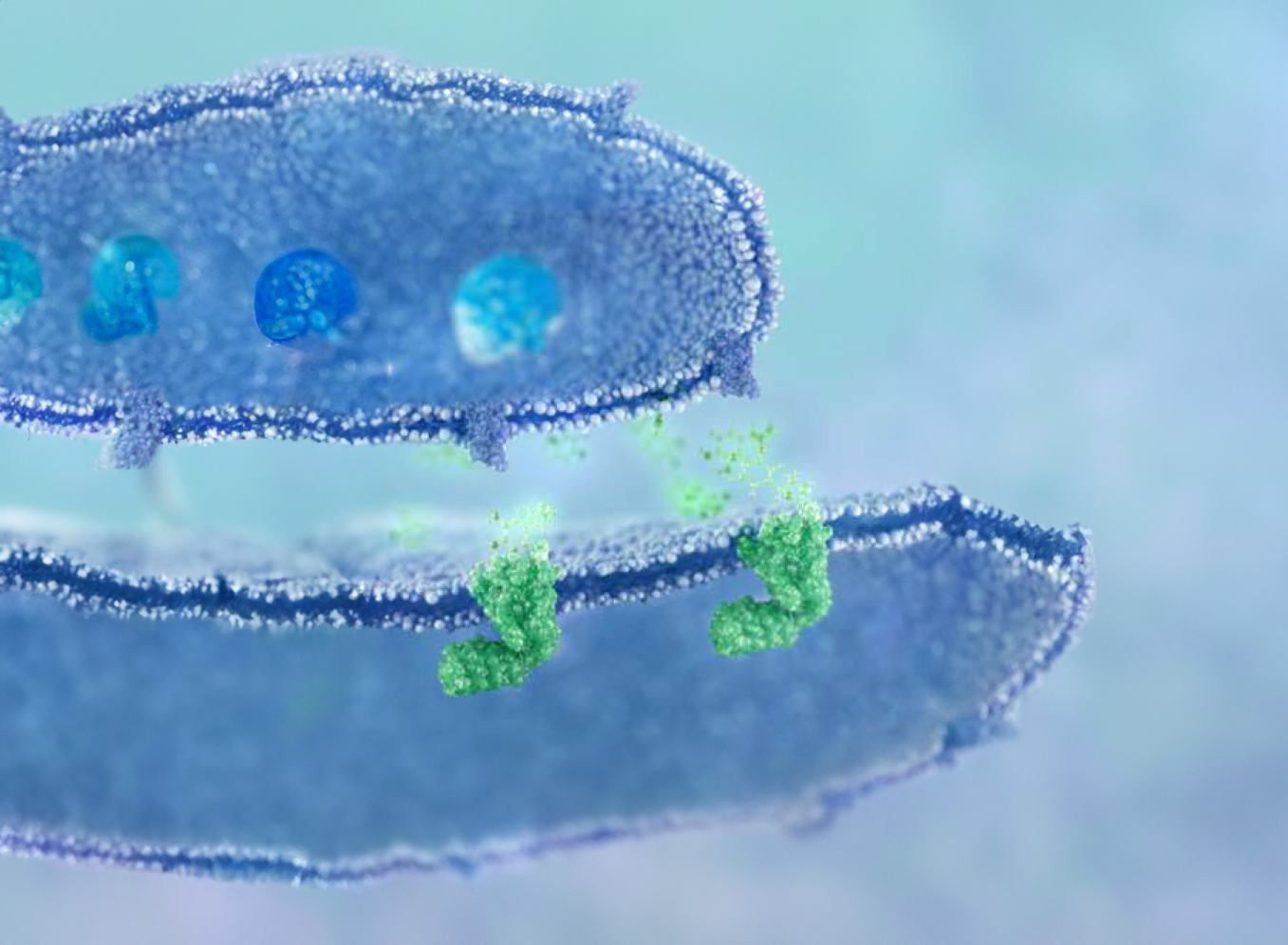
AD109 is an investigational first-in-class combination of aroxybutynin, a novel antimuscarinic, and atomoxetine, a noradrenaline reuptake inhibitor (NRI).
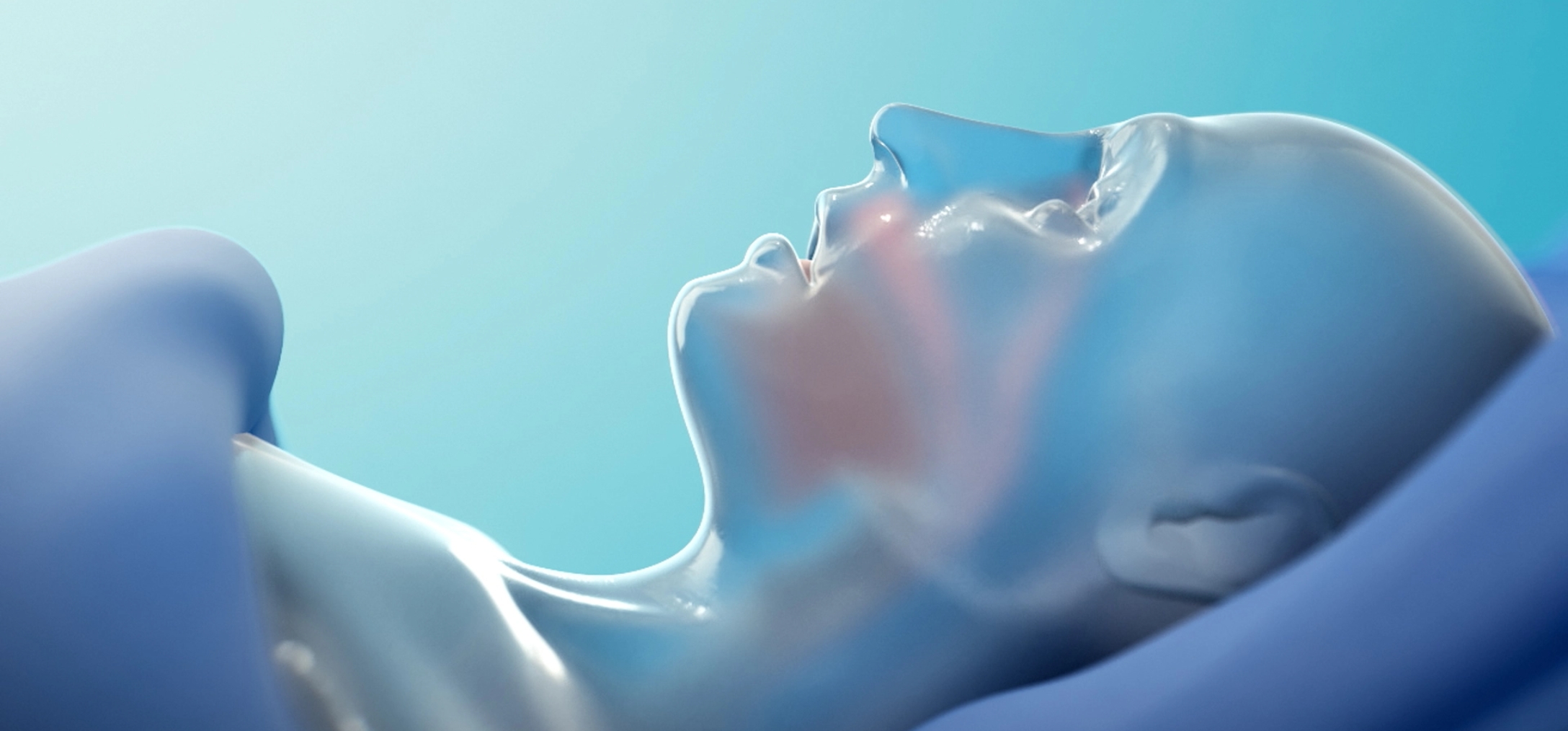

By targeting the underlying neuromuscular dysfunction of OSA, AD109 offers an innovative therapy that has the potential to improve the lives of millions of people living with this serious chronic disease.
Hoyee Leong
PhD, Vice President, Medical Affairs

A Simple Solution
for Oxygenation
AD109 represents potential new hope for people living with OSA who have either refused, abandoned, or are currently dissatisfied with their treatment.
The investigational therapy is a simple once-nightly oral pill that lowers the complexity of intervention and may help more people benefit from effective, restorative sleep.

AD109 Clinical Program
We have conducted more than 15 Phase 1 and 2 clinical trials that inform various aspects of the AD109 program and have amassed efficacy, safety, and tolerability data for AD109 across multiple completed clinical trials in OSA.
In the pivotal MARIPOSA Phase 2b clinical trial, the study results showed that AD109 at both doses tested achieved a statistically significant reduction of AHI compared to placebo (P<0.001 vs placebo).
As an endpoint, AHI is the most used metric to diagnose and assess the severity of OSA. It takes into consideration the number of apneas (when breathing completely stops) and hypopneas (breathing with greatly reduced airflow) during each hour of sleep.
AD109 is also currently being studied in two large, pivotal Phase 3 randomized clinical trials, LunAIRo and SynAIRgy, for mild, moderate, and severe OSA.


Stay Updated
Submit your email for Apnimed company news, including updates on AD109.


Explore Our Full Pipeline
AD109 is only the beginning.
